A Comprehensive Integrator of Technology, Industry and Trade
We integrate R&D, manufacturing, and global trade to deliver innovative products and exceptional value.
⛟ No.11 Chengen Road, Pubagang Town, Sanmen County, Zhejiang Province, China.
QUALITY CERTIFICATIONS
Third-party verified excellence in
nutritional health solutions.

FDA

PMDA

ISO 9001:2015

CNAS

Ministry of Food and Drug Safety.

European Medicines Agency
Science Medicines Health.

National Medical Products Administration.
ABOUT US
We always delivers expertise and
compassion in every interaction.

Established in 2008, Zhejiang Hengkang Pharmaceutical Group is a dynamic pharmaceutical entity, spanning drug research, large-scale production, and global marketing. With an emphasis on high-end active pharmaceutical ingredient R&D, manufacturing, and sales, Hengkang is devoted to delivering superior pharmaceutical products and professional services.
Headquartered in Hangzhou, Hengkang established multiple factories, research and sales centers in Zhejiang, Shandong, and Henan. With facilities conforming to global API standards, along with professional R&D and marketing teams, Hengkang’s operations span major pharmaceutical markets such as China, Europe, the CIS region, Japan, and South Korea. Hengkang has established long-term and stable relationships with nearly 70 countries overseas and hundreds of domestic enterprises.
CMO Service Why Choose Us
Precision Biosynthesis Experts: Fermentation Tech, Healthcare Ingredients, Enzyme Protein, Medical Beauty Products, Synthetic Biology & Beyond
20+ Years of Production Expertise
Proven experience in biopharmaceutical manufacturing with a track record of quality and reliability.
60+ CDMO/CMO Service Projects
End-to-end contract development and manufacturing support, from preclinical to commercial-scale production.
cGMP-Compliant Pharmaceutical System
Strict adherence to Current Good Manufacturing Practices (cGMP), ensuring global regulatory compliance.
Sales Network in 70+ Countries, 400+ Clients
A robust global distribution network, delivering products to partners and patients worldwide.
Backed by Industry Investment Funds
Strong financial and strategic support, enabling innovation and scalable growth.

Together Towards Tomorrow: Partnering for Your Health and Wellness Journey!
CDMO SERVICES
Dedicated to new drug R&D and the production of
APIs and intermediates.

Process R&D:
· Customized chemical synthesis.
· Scale-up synthesis.
Pharmaceutical Research:
· API process route selection and optimization.
· Impurity studies.
· Salt/Polymorph screening.
· Pre-formulation studies.
· Stability studies.
· Drug product and process studies.
· Registration and application.
Advantages of Our APIs
Your Reliable API Supplier With
Good International GMP&Excellent Service
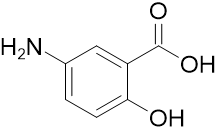
Product Name: Mesalazine / Mesalamine
CAS: 89-57-6
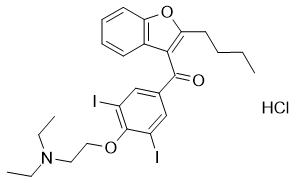
Product Name: Amiodarone HCl
CAS: 19774-82-4
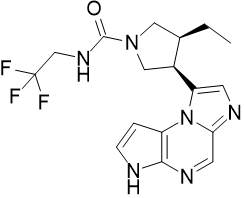
Product Name: Upadacitinib
CAS: 1310726-60-3
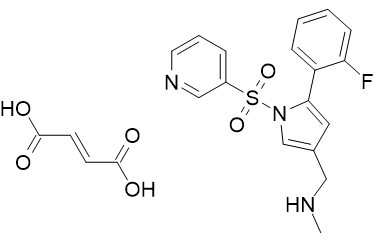
Product Name: Vonoprazan Fumarate
CAS: 1260141-27-2
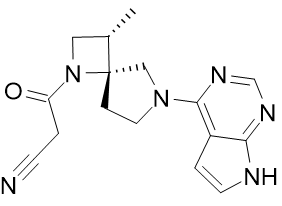
Product Name: Delgocitinib
CAS: 1263774-59-9
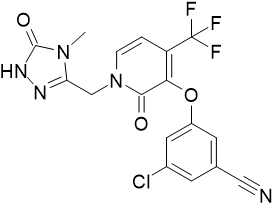
Product Name: Doravirine
CAS: 1338225-97-0
